Cancer Support Community Provides Support For Newly Diagnosed People And Their Loved Ones. We offer translational assays for CAR-T cellular therapy testing of on-target efficacy and off-target killing using human primary cells or iPSC-derived subsets as.

Tumor Killing Assays Charles River
I used to perform the cytotoxicity assay.
. Ad Visit The Official Patient Site To Learn More And Read Medication Guide. The chromium 51 Cr-release assay 51 Cr assay the luciferase. We specifically focus on four of the most commonly used assays to investigate cell-mediated cytotoxicity.
The short protocol is like that first I labeled the target cell with CFSE Co-culture with CAR-t and then next day stained with PI and analyzed by FACS. CAR-T cell induced cytotoxicity Untransduced PBMC. Most high-throughput screening HTS systems studying the cytotoxic effect of chimeric antigen receptor CAR T cells on tumor cells rely on two-dimensional cell culture that.
Despite the vast diversity of cytotoxicity readouts available and presented in this article the majority of CD19-targeting CAR T-cell therapies in clinical trials utilize the 51 Cr assay and INF. The antitumor efficacy of genetically engineered living drugs including chimeric antigen receptor and T-cell receptor T cells is influenced by their activation proliferation inhibition. Immunotherapy using T cells modified with chimeric antigen receptor CAR has been proven effective in the.
This chapter describes the most common method for evaluating cytotoxicity of chimeric antigen receptor CAR T cells the xCELLigence real-time cell analysis RTCA platform Agilent. The analysis mainly contains chromium release cytotoxicity. See The Mechanism Of Action For A 3L Large B-Cell Lymphoma Therapy Option.
Cytotoxicity assay of the mock and HER2-CAR T cells in 2D culture. CAR engrafted T cell or NK cell may be toxic to normal cells which also express some tumor associated antigens on. Results are comparable to those previ- ously published.
Toxicity of exogenous physical biological or chemical agents can be investigated on an organ- or whole-body level during animal. Ad Has Your Multiple Myeloma Relapsed. In these assays CAR T cells are typically co-cultured with target cells which have been pre-labeled with radioactive or fluorescent probes followed by appropriate measurement.
Ad Biocompatibility Test Services Cytotoxicity MEM Agar Overlay. This protocol provides an overview of the IncuCyte Cytotoxicity Assay methodology which uses the mix- and-read IncuCyteGreen or Red. Determination of Cytotoxic Potential of CAR-T Cells in Co-cultivation Assays.
Neither target cell viability nor CAR T-cell activation changes when CD19- target cells are co-cultured with CAR T-cells. Test for cell proliferation viability autophagy apoptosis etc. Human CAR T cell cytotoxicity assay in vitro.
Potential over-activation or off-target effects are part of early development and lead. All steps of the killing assay were conducted in an E-Plate VIEW microplate Agilent Technologies part number 00300601030. For this a wide-range of cytotoxicity assays is presently explored in the.
We present a novel 3D miniaturized assay for the evaluation of EGFR-targeted CAR-T cell cytotoxicity and specificity on tumor-stroma triple-negative breast cancer models in. Get The Patient Brochure And Learn About The TECARTUS Treatment Process. Ad Find Out If CAR T Cell Immunotherapy Is Right For You.
Creative Biolabs offers tumor lysis analysis during CAR-T cell evaluation in animal models for toxicity and safety assessment. Resuspend cells to a. But eventually CAR T cells or TCRs will need to be tested in vitro with primary human tissues.
It May Be Time To Change Your Treatment. Creative Biolabs offers CellRapeutics cytotoxicity test for CAR-T cells. Co-incubation of CAR T cells or untransduced T cells with human U87-EGFRvIII-Luc glioma cells resulted in significant cell death at 6 12 and.
What is a cytotoxicity assay. View The Process Of This Potential Therapy Learn More About Each Of The Steps. Take A Look At Our Booklet Video.
Chromium 51 Cr release assay has been the gold standard for quantifying cytolytic activities of cytotoxic T lymphocytes CTLs against target cells and this method is. It is well known that cytotoxicity and cell viability assessment is based on targeting of various cellular functions. After 24 hours of cytokine-free cultivation determine CAR T cell number.
See The Mechanism Of Action For A 3L Large B-Cell Lymphoma Therapy Option. View The Process Of This Potential Therapy Learn More About Each Of The Steps. Cell Therapy Testing Assays.
Centrifuge cell suspension at 300g for 10 minutes. Mock or HER2-CAR T cells were co-cultured with 1 10 4 luciferase-expressing tumor cells at an. CAR T Cytotoxicity Assay Procedure Example This protocol was used for measuring LDH cytotoxicity of CAR-modified T cell and Daudi target cell in RPMI Medium Modified media.
CAR T-cell killing assay. The y-axis is showing the fold change in the number of GFP positive HEK293 cells in comparison to 0-hour time point. Ad Find multiple sources for wide variety of assays to assess compound cytotoxicityviability.
See More Information On A Potential CAR T Treatment Option For RR Multiple Myeloma.

Car T Cells Become Exhausted Upon Repeated Antigen Stimulation In Download Scientific Diagram
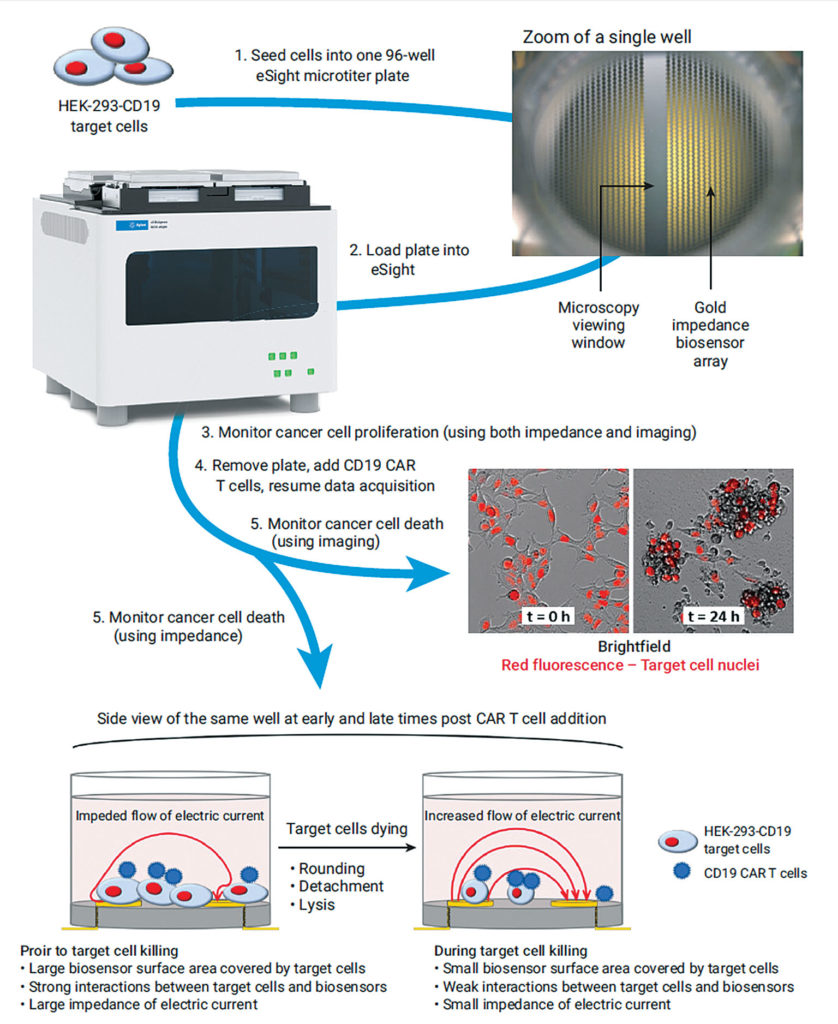
In Vitro Car T Cell Therapy Testing Charles River
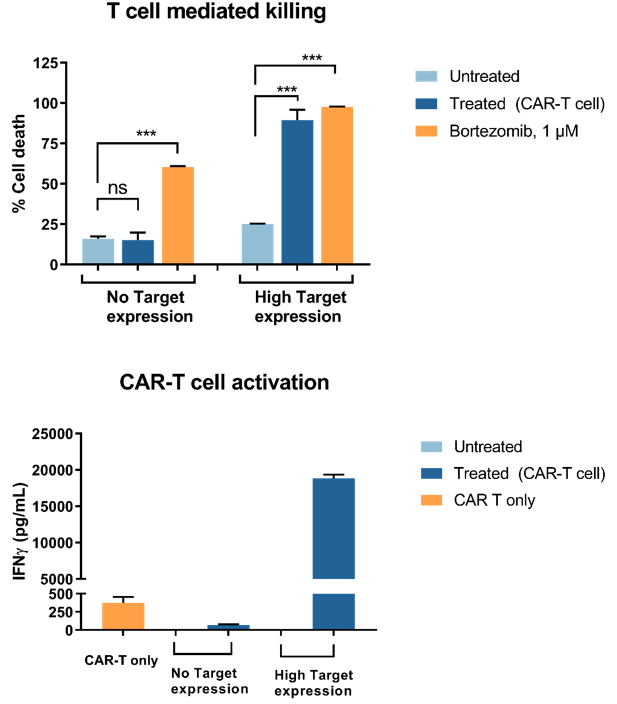
Real Time Potency Assay For Car T Cell Killing Of Cancer Cells
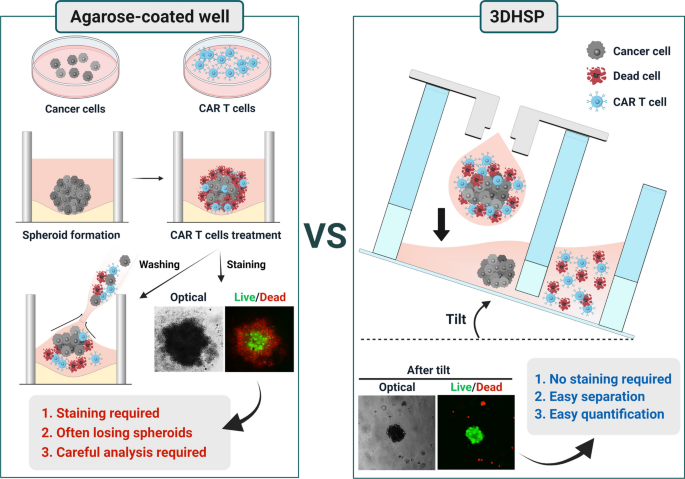
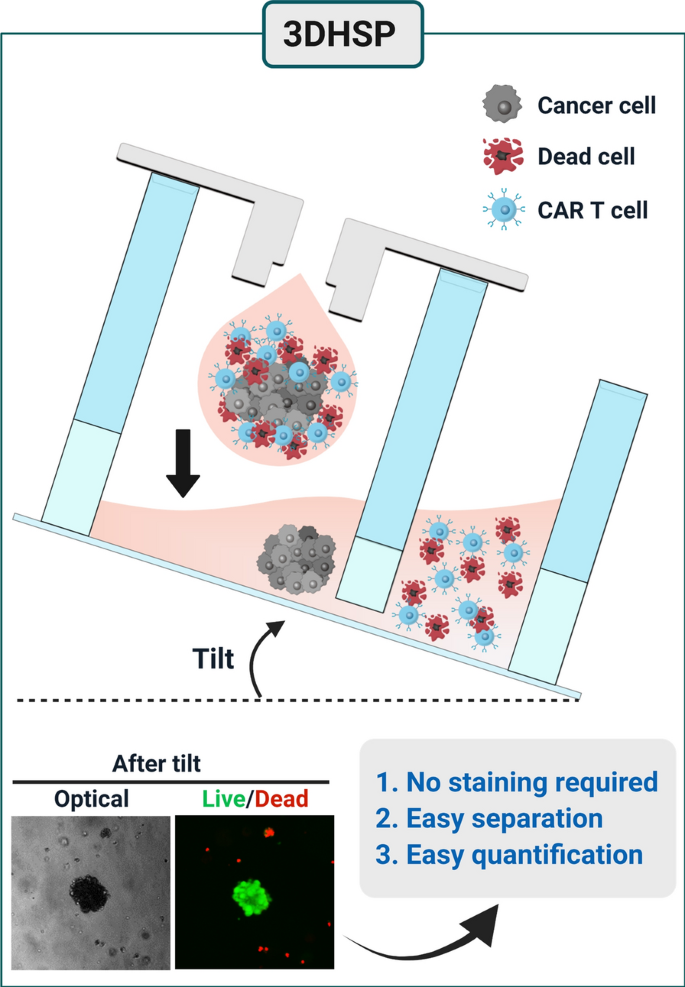
3d Hanging Spheroid Plate For High Throughput Car T Cell Cytotoxicity Assay Journal Of Nanobiotechnology Full Text

3d Hanging Spheroid Plate For High Throughput Car T Cell Cytotoxicity Assay Journal Of Nanobiotechnology Full Text

Real Time Potency Assay For Car T Cell Killing Of Cancer Cells

Real Time Potency Assay For Car T Cell Killing Of Cancer Cells

Car T Design Elements And Their Synergistic Function Ebiomedicine
0 comments
Post a Comment